Elements Described in Terms of their Atoms?
Lesson 3.1
Key Concepts
Key Terms
- How are elements described in terms of their atoms?
Key Terms
- protons
- neutrons
- electrons
- atomic number
- isotopes
- mass number
- model
How are elements described in terms of their atoms?
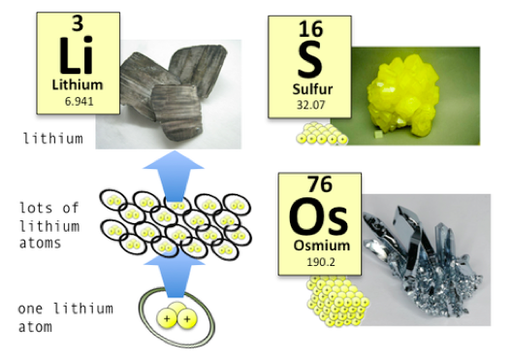
Number of Protons
- Each element is made up of atoms that differ from the atoms of other elements.
- An element can be identified by the number of protons in the nucleus of its atoms.
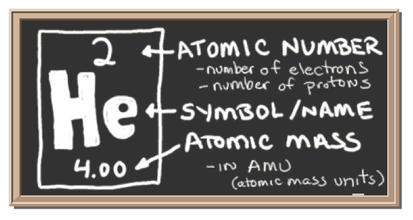
Atomic Number
- Every atom of an element has the same number of protons. This unique number is called the atomic number.
- It is equal to the number of protons in the nucleus of an atom.
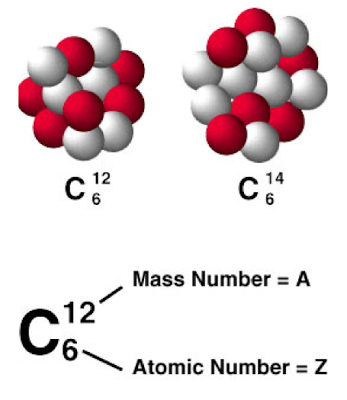
Isotopes
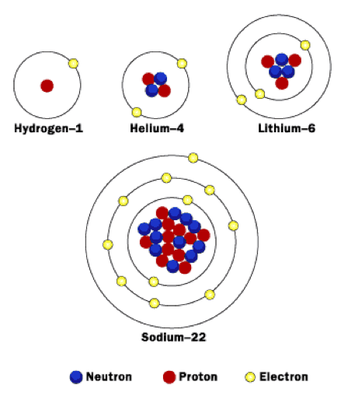
- Although all atoms of an element have the same number of protons, they may have different numbers of neutrons.
- Isotopes are atoms with the same number of protons and a different number of neutrons.
Mass Number
- An isotope is identified by its mass number, which is the sum of the protons and neutrons in the nucleus of that atom.
- Although isotopes have different mass numbers, they react the same way chemically.