What are the Properties of a Mixture?
Describing Matter Lesson 1.1
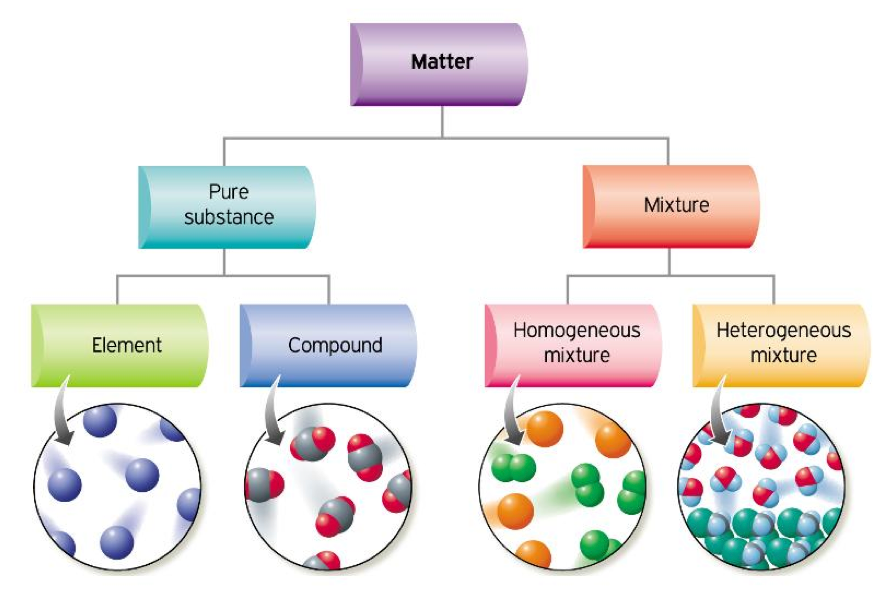
Pure Substances & Mixtures
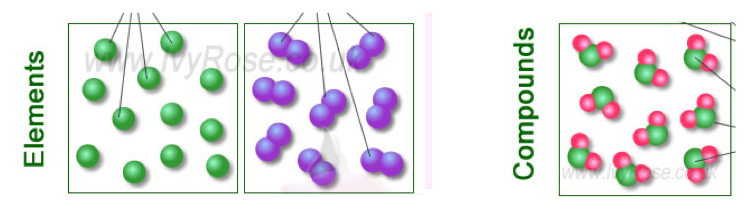
- Elements and compounds are pure substances, but most of the materials you see every day are not.
- Instead, they are mixtures.
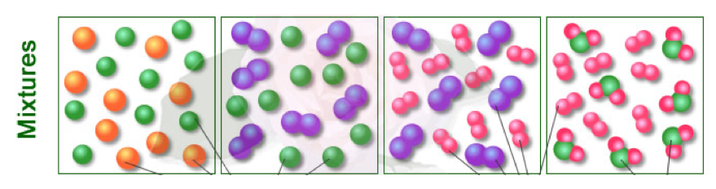
Mixtures
- A mixture is made of two or more substances that are together in the same place, but are not chemically combined.
- Each substance in a mixture keeps its individual properties.
- The parts of a mixture are not combined in a set ratio.
Hetero & Homo Geneous

Heterogeneous
- In a heterogeneous mixture, you can see the different parts.

Homogeneous
- The substances in a homogeneous mixture are so evenly mixed that you cannot see the different parts.
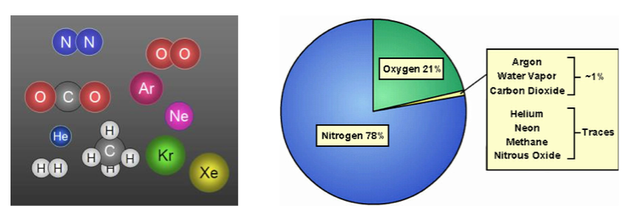
Solution
- A solution is an example of a homogeneous mixture.
- Air is a solution of nitrogen, oxygen, plus small amounts of other gasses.
Mixtures
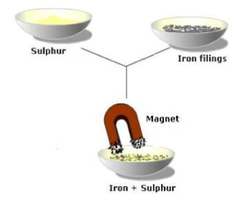
- Unlike compounds, mixtures are easily separated into their components.
- For example, iron filings can be easily removed from salt with a magnet.