What are ELEMENTS, and how do they relate to compounds?
Describing Matter Lesson 1.1

Elements
- All matter is made up of elements.
- An element is a pure substance that cannot be broken down into any other substance.
- Elements are the simplest substances.
- Each element is identified by its specific physical and chemical properties.
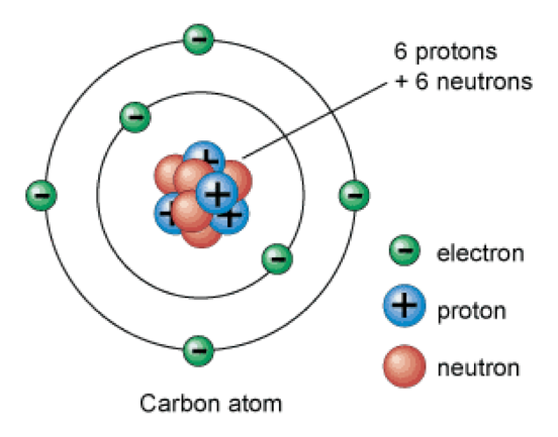
Atom
- An atom is the basic particle that makes up an element.
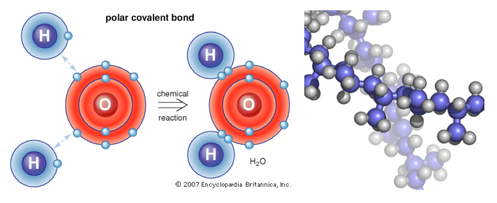
Chemical Bond
- A chemical bond is the force that holds two atoms together.
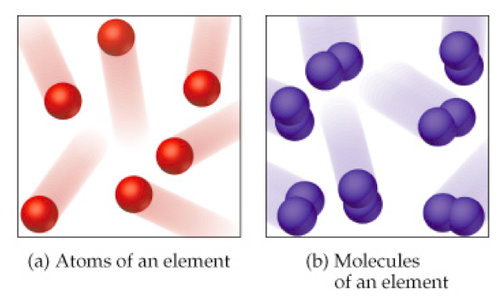
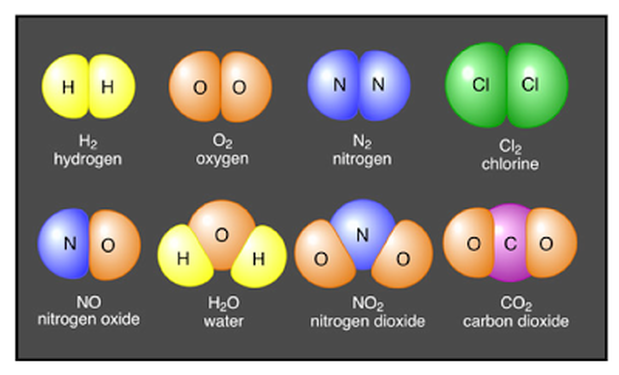
Molecules
- Atoms often combine to form molecules, which are larger particles made of two or more atoms held together by chemical bonds.
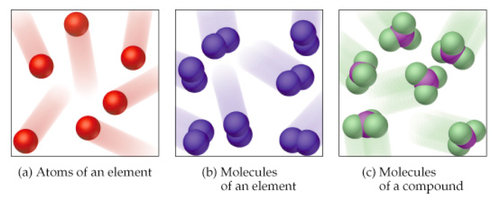
Compounds
- When elements are chemically combined, they form compounds having properties that are different from those of the uncombined elements.

Chemical Formula
- A compound may be represented by a chemical formula.
- A chemical formula shows the elements in the compound and the ratio of atoms.
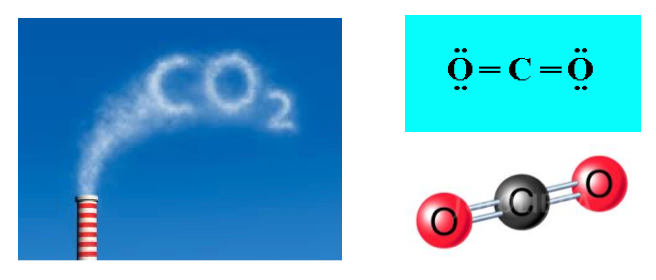
- For example, the chemical formula for carbon dioxide is CO2.
- In carbon dioxide, there are always two oxygen atoms for every one carbon atom